Lithium iron phosphate production line
Lithium iron phosphate is mainly used as a positive electrode material for lithium-ion batteries. According to the different electrolyte materials used in lithium-ion batteries, lithium-ion batteries can be divided into two categories: liquid lithium-ion batteries and polymer lithium-ion batteries. It mainly includes lithium cobalt acid battery, lithium nickel acid battery, lithium manganese acid battery, lithium iron phosphate battery and so on. Among them, lithium iron phosphate has a huge advantage in the production of high-power lithium batteries, and its raw iron source, lithium source and phosphorus source are conventional chemical materials, and will not cause environmental pollution.
Main processes: high temperature solid phase method, carbothermal reduction method, liquid phase coprecipitation method
Main raw materials: iron source, lithium source, phosphorus source
Main use: lithium ion battery cathode material
Outstanding advantage
Experimental analysis: The company's pilot test line can carry out a variety of raw material analysis, formula design, sample preparation, roasting, pilot test, pilot test.
Environmental protection and high efficiency: the use of fully closed air transport system, generating no dust pollution throughout the process.
Advanced process equipment: from the overall project planning to the detailed process formula, process design, equipment design and manufacturing, engineering installation, commissioning, etc., to provide a complete whole industry chain services, with a complete solid hazardous waste treatment system solutions.
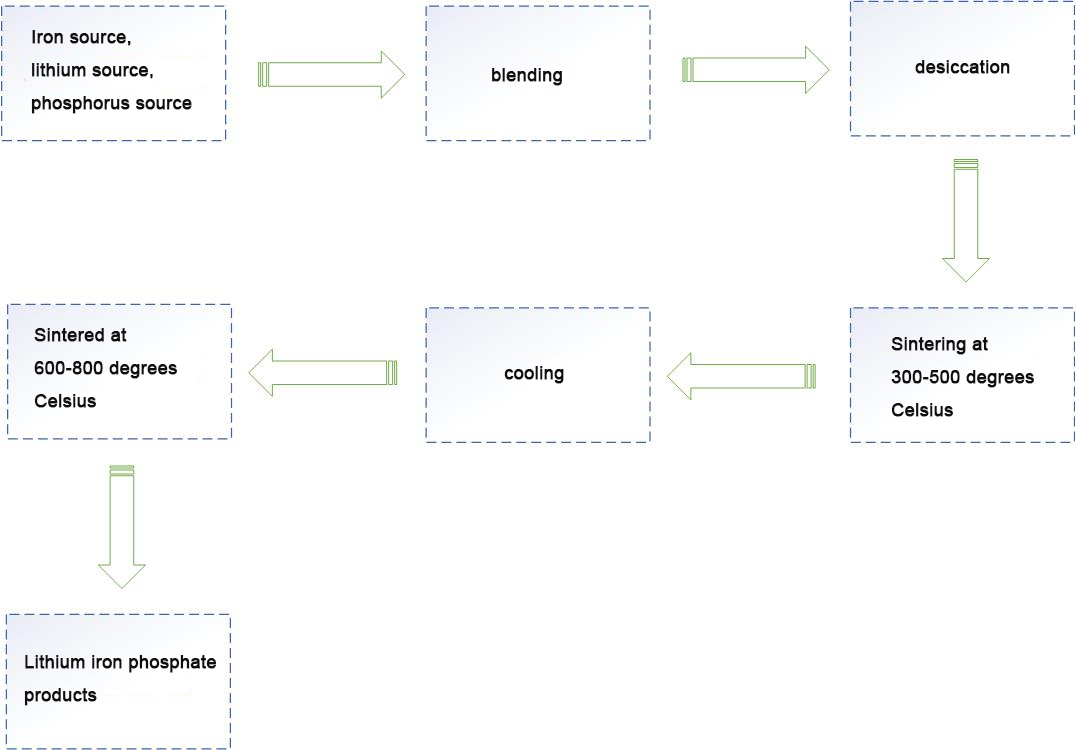
Process flow
I. High temperature solid phase method
High temperature solid phase method mainly adopts solid phase synthesis method. Lithium carbonate and lithium hydroxide are used as lithium sources, ferrous oxalate, ferrous oxalate, iron oxide and iron phosphate are used as iron sources, and the phosphate group mainly comes from ammonium dihydrogen phosphate and so on. The typical process flow is: after the raw material is ground and dried, it is heated to a certain temperature at a certain temperature acceleration in an inert or reducing atmosphere in a Muffle furnace or tube furnace, and then cooled after a period of reaction. The advantages of high temperature solid phase method are simple process and easy to achieve industrialization, but the particle size of the product is not easy to control, the distribution is not uniform, the morphology is irregular, and the inert gas protection is required in the synthesis process.
II. Carbothermal reduction method
This method is an improvement of the high temperature solid phase method, which directly uses the high price oxides of iron such as FeO, LiHPO and toner as raw materials, mixed with a stoichiometric ratio, sintered at 700℃ in the argon atmosphere of the box-type sintering furnace for a period of time, and then cooled to room temperature naturally. HPO and FeCL synthesize FePO.2HO, which is then combined with CHCOOLi to hydrothermal synthesize LiFePO. Compared with the high temperature solid phase method, the temperature of hydrothermal synthesis is low, about 150 degrees ~ 200 degrees, the reaction time is only about 1/5 of the solid phase reaction, and lithium iron phosphate can be directly obtained, no inert gas is required, the product grain is small, homogeneous phase and other advantages.
III. Liquid coprecipitation method
Through the use of a large number of organic complexers, the sol/gel method can make lithium, iron and phosphorus evenly distributed at the atomic or molecular level, but this preparation method is expensive and difficult to scale production.


































