Soda Ash Production Line/production Plant/turnkey Project/sodium Carbonate Production Equipment/process
I. Introduction to production technology
Soda ash is one of the important chemical raw materials. It is widely used in glass, daily chemistry, chemicals, enamel, papermaking, medicine, textiles, textiles, printing and dyeing, and other industrial departments, as well as people's daily lives, and occupy an important position in the national economy.
1. Craft plan selection
•There are three main methods for production of pure alkali production: natural alkali processing, aminine method, and combined alkali method (Hou's alkali method). There is also a Lublan method that has been eliminated: first use sulfuric acid and sodium salt sulfate, and then mix sodium sulfate, limestone and coal to burn in the rotary kiln to generate melting blocks, then immersed, evaporated, burning, etc. And make pure alkali. Lublanfa has poor production continuity, small equipment production capacity, severe equipment corrosion, poor labor environment, poor product quality, low income, and insufficient raw materials.
•Ammonia-alkali process (Solvay process) is one of the general industrial methods for large -scale pure alkali in the world in the world. After more than 100 years of production practice test, its production technology has matured and stable technology, and has achieved continuous operations, large production capacity, and high product purity.
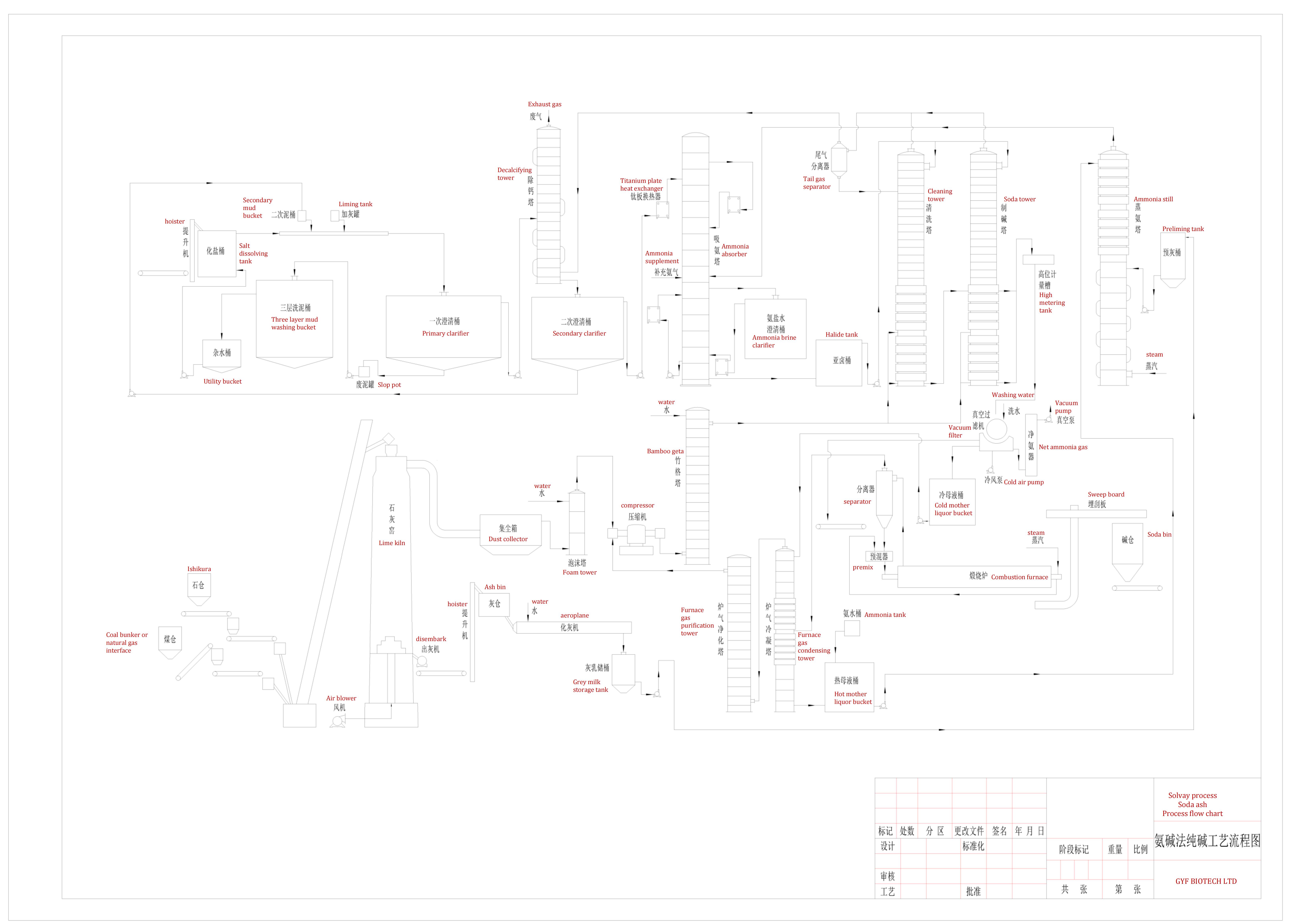
•Solvay process is a complex chemical manufacturing process. It mainly includes a series of chemical unit operations. A total of nine processes: lime processes, saline processes, ammonia suction processes, carbon chemical processes, filtration processes, steamed ammonia processes, compression processes , Burning process, packaging process.
The main raw materials of ammonia -alkali production: limestone, salt, scorched carbon, ammonia, etc.
1.2 Process scheme selection
•In this project, light soda ash is produced by Solvay process method .
•The main raw materials : limestone, salt, cokes, ammonia, etc
2. Craft production basis
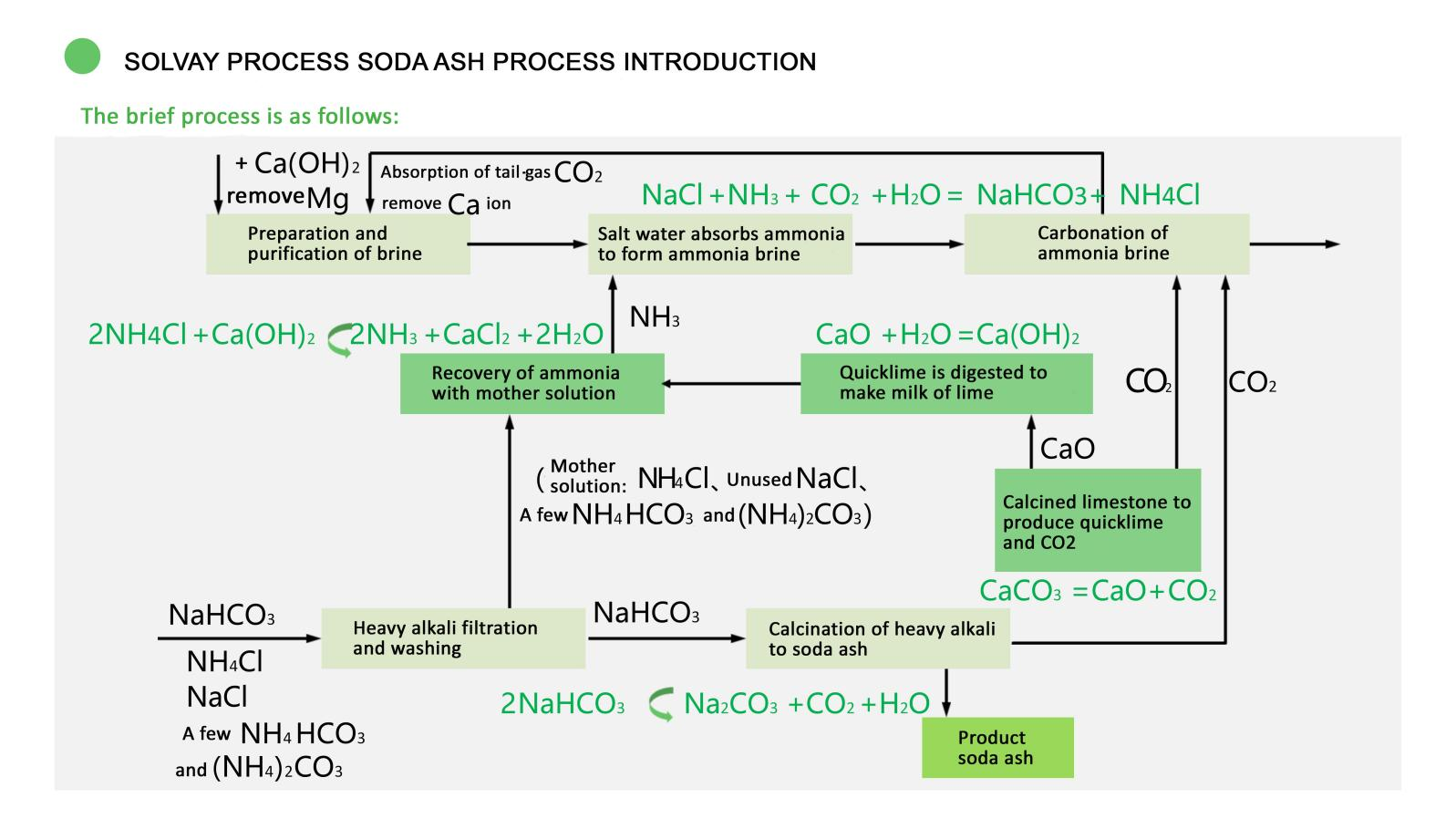
①Limestone burning to make carbon dioxide and calcium oxide (lime)
CaCO3 = CaO+CO2
Carbon in the fuel burns in the air to generate carbon dioxide and heat up
C+O2 = CO2+Q
Calcium oxide (raw lime) digestion to make mature lime
CaO+H2O = Ca (OH) 2
②Saturated salt water absorption and carbonization into heavy alkali
NaCl+NH3+CO2+H2O = NH4CL+NaHCO3
③Separate the heavy alkali from the solution, the burning is burned, and the soda is made, and the carbon dioxide is recovered
2NaHCO3 = Na2CO3+CO2+H2O
④The NH4+ and several ammonium carbonate salts in the solution are decomposed and then the ammonia is steamed out for recycling
2NH4Cl+Ca (OH) 2 = 2NH3+CaCl2+2H2O
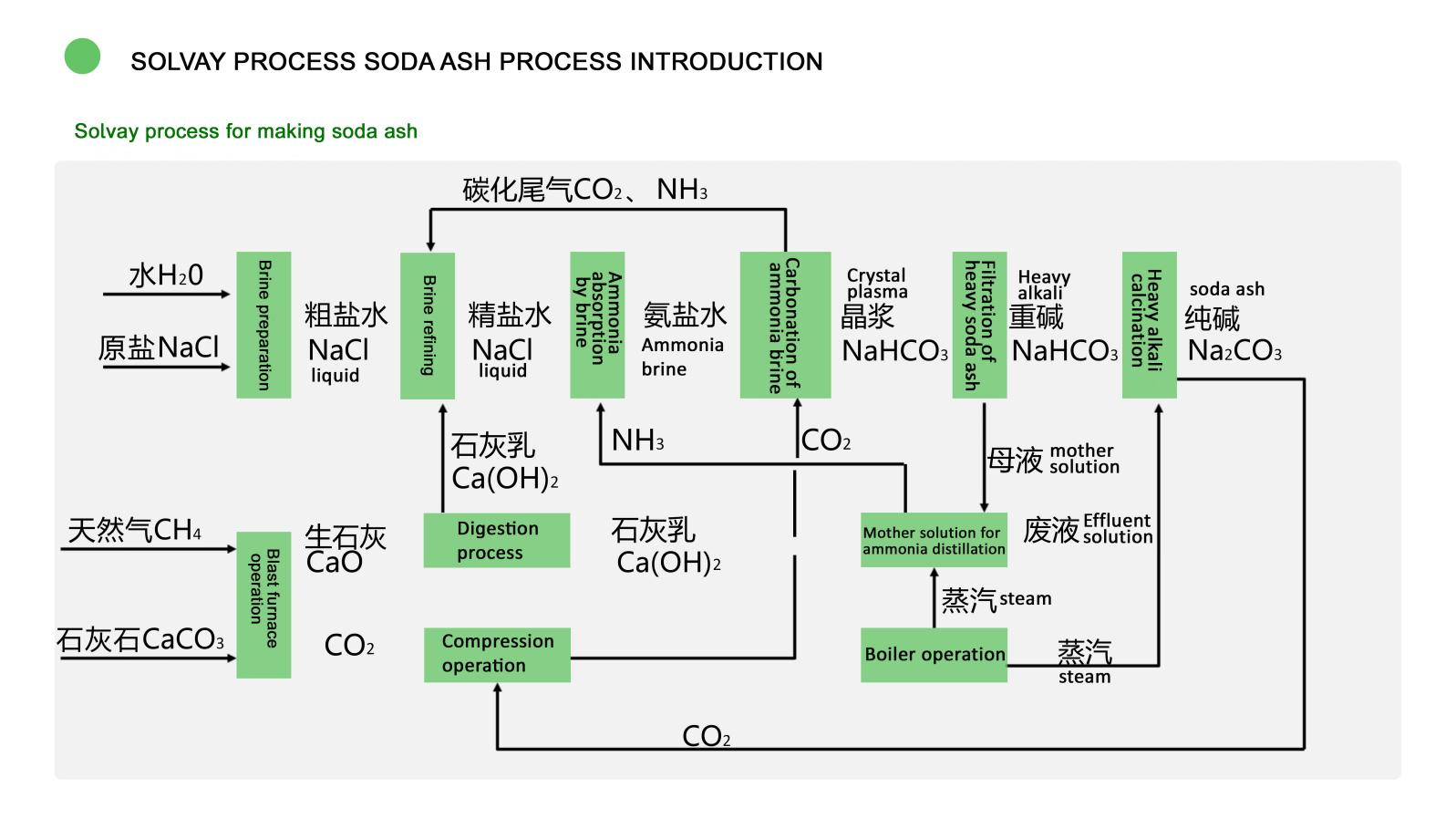
3. Brief description of process flow
Solvay production process:
- First, the raw salt is dissolved with water to produce saturated coarse salt water, and then the impurities are removed by lime soda method or lime ammonium carbonate method to obtain refined salt water.
- The refined salt water is cooled in the ammonia absorption tower and the ammonia steamed by the ammonia distillation tower countercurrent absorption to make ammonia brine. After cooling, the ammonia brine reacts with carbon dioxide in the carbonization tower to produce sodium bicarbonate. The suspension with crystallization is pressed out from the bottom of the tower and sent to the vacuum filter to separate the heavy alkali.
- The filtered heavy alkali filter cake is heated and decomposed in the calcination process to produce light soda ash and furnace gas, light soda ash is cooled and packaged into commercial light soda ash. If the light soda ash is sent to the hydration machine through the transport equipment, the heavy soda ash can be produced.
- The carbon dioxide escaping from the decomposition process is separated, cooled and purified, and then pumped and compressed by the compressor to return to the carbonization process.
- The filter mother liquor extracted by the vacuum filter is sent to the ammonia distillation tower and reacts with the lime milk obtained by calcination, decomposition and digestion of limestone to steam out ammonia, and returns to the ammonia absorption tower for recycling.
- The ammonia waste is discharged into the slag yard.
- Lime is produced by calcination of coke in lime kiln, and then reacted with water by chemical aircraft to make lime milk, which is sent to the ammonia distillation process and the brine process respectively.
- Lime kiln gas with 40% carbon dioxide and calciner gas with more than 80% carbon dioxide are used through the compressor carbonization process.